HCOOCH CH2 H2O is consist of formic acid(HCOOH), methylene group(CH2), and water(H2O). The vinyl formate (HCOOCH=CH2) and water(H2O) is used in various reactions such as hydrolysis, polymerization, electrophilic addition to vinyl group, and oxidation reaction.It is used in industry to create polymers, in search department it is used to study ester hydrolysis, and also to study chemistry of vinyl compound.
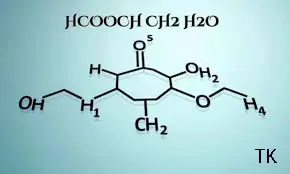
Key components of HCOOCH CH2 H2O
There are three main chemical components present in it.
- Formic acid(HCOOCH): The carboxylic acid present in formic acid is often used as acid catalyst.
- Methylene unit(CH2): Methylene unit have two electrons that are involve in polymerization.
- Water(H2O): The water molecule transfer its proton in hydrolysis, and hydration process.
The partial hydrolysis of vinyl formats result in the formation of formic acid and vinyl alcohol.
Molecular structure of HCOOCH CH2 H2O
Its molecular structure is consist of vinyl formate and water molecule.
1.Vinyl Formate (HCOOCH=CH₂)
O
||
H–C–O–CH=CH₂
The hydrolysis of vinyl formate result in the formation of formic acid and vinyl alcohol.
HCOOCH=CH2 +H2 O⟶HCOOH+CH2 =CH–OH
HCOOH is the formic acid and CH2 =CH–OH is the vinyl alcohol.
2.Water(H2O)
The water molecule is the polar part of HCOOCH CH2 H2O.
H–O–H
The water molecule is act as a nucleophile in hydrolysis of vinyl formate. It also donate a lone pair of electrons in hydrolysis.
Reactions of HCOOCH CH2 H2O
Hydrolysis
The hydrolysis of ester bond is the primary reaction between vinyl formate and water molecule.
In ester hydrolysis, H2O attacks the ester bond and result in the formation of formic acid and vinyl alcohol.
HCOOCH=CH2 +H2 O→HCOOH+CH2 =CH–OH
The vinyl alcohol that is formed in the result of hydrolysis is unstable, so rearrange it into acetaldehyde. This step is called tautomerization.
CH2 =CH–OH→CH3 –CHO
So formic acid(HCOOCH) and acetaldehyde(CH3CHO) are the final product of hydrolysis.
HCOOCH=CH2 +H2 O→HCOOH+CH3 CHO

Polymerization
The vinyl formate part of HCOOCH CH2 H2O undergo radial polymerization. This reaction is not directly perform with water because if water is present, it may cause chain transfer and also limit the polymerization.
n⋅HCOOCH=CH2 →[–CH₂–CH(HCOO)–]n
Acid or Base catalysis
The water is act as a medium for transfer of proton. Due to presence of water the hydrolysis occur faster.
Applications
Used as solvent
It is used as solvent in chemical reactions and synthesis of glycol derivatives polyesters, and formate ester. It also acts as primary component in organic synthesis of complex molecules. Moreover, hydroxyl group participants in polymerization for the production of polyesters and polyurethanes.
Lubricant and decomposition agent
It act as a building block in lubricant formation and plasticizer. Esters like hydroxy-ethyl formate have potential in fuel cell or hydrogen storage applications, if they are decompose into CO2 and H2. Esters like formic acid are explored for controlling the release of hydrogen.
Pharmaceutical and electronic applications
It may also used in the synthesis of active pharmaceutical ingredients like APIs. Moreover, it is used as solvent in electronics. It is also used as cleaning agent due to its solvent property.
Lab technique with HCOOCH CH2 H2O
Esterification
Under acidic condition formic acid react with ethylene glycol to form esters.
HCOOH+HOCH2 CH2 OHH+ HCOOCH2 CH2 OH+H2 O
In the presence of acidic catalyst, carboxylic acid react with alcohol. The yield is depend upon purity of reagents and reaction time.
Purification technique
In liquid-liquid extraction, it use polar or non-polar solvent to separate the product.
Hydrolysis
It undergo hydrolysis to yield formic acid and ethylene glycol.
Trans-esterification
Under acidic/basic condition it react with alcohol to form esters.
Safety measures
Here are some safety measures for working with it in laboratory settings.
- Use gloves, lab coats, long sleeves for protection.
- Avoid high heart or open flame.
- It may cause irritation, so use soap.
- It may also cause irritation or redness in eye, so rinse your eyes for 15 minutes with water.
- Do not breathe vapours or mist which may cause irritation in nose, throat, lungs, so move to fresh air or medical treatment.
- Keep distance from acids and oxidizing agents.
- Use sealed bottles to reduce hydrolysis.
Conclusion
HCOOCH CH2 H2O is a valuable ester that is formed by the combination reaction of ethylene glycol and formic acid. It is important in industry, laboratory, and also in polymer production and organic synthesis due to its dual nature. In laboratory through Fischer esterification, we form esters by reacting formic acid with ethylene glycol.
Due to its irritant property and flammability, proper storage and safety measures are required. For proper characterization of HCOOCH CH2 H2O analytical techniques like IR, NMR are helpful. It is useful in research and industrial chemical engineering due to its multi-functionalty.
FAQs
Q#1 Write formation of HCOOCH CH2 H2O?
HCOOCH CH2 H2O is formed by the combination of formic acid(HCOOH), methylene group(CH2), and water(H2O).
Q#2 Write reactions of HCOOCH CH2 H2O?
HCOOCH CH2 H2O hydrolysis result into formation of formic acid and vinyl alcohol.
HCOOCH=CH2 +H2 O→HCOOH+CH3 CHO
Furthermore, the vinyl part undergo polymerization.
n⋅HCOOCH=CH2 →[–CH₂–CH(HCOO)–]n
Q#3 Why safety measures are important?
Due to its irritation and flammable property the safety measures like use of gloves, lab coats, avoid direct contact to acids, use of sealed bottles, and avoid high heat are important.
Q#4 Write molecular structure of HCOOCH CH2 H2O?
It is a combination of Vinyl Formate (HCOOCH=CH₂) and water (H2O).
Vinyl Formate (HCOOCH=CH₂) Water (H2O)
O
|| H-O-H
H–C–O–CH=CH₂
